I came across an interesting new product recently which made me realize that I take batteries for granted without actually thinking much about how they work. I know they work and the specifications enable me to select them and design them into a product but how do they work? The product that caused my intrigue was the salt water powered lamp being developed in the Philippines salt.ph. It is powered for 8 hours using water and a couple of tablespoons of salt or using sea water. I won’t get into the arguments about whether it is “sustainable” or environmentally friendly” – it just piqued my interest to know more about how batteries work.
In the past you may have seen potato or lemon batteries as gimmicks or educational aids. They rely on the choice of materials for the two electrodes in order to work. Just inserting two copper wires into a potato won’t work (but one copper and one zinc electrode would). A battery is often used to describe a collection of cells to give a higher voltage than a single cell would achieve although that is not strictly adhered to.
A primary cell is one that cannot be recharged. You use it once then throw it away. When using a primary cell there is a chemical reaction going on and it cannot be reversed by simply reversing direction of current flow. The Leclanche cell of 1866 was a zinc-carbon primary cell. The additional materials used were manganese dioxide and an electrolyte of ammonium chloride. The manganese dioxide is a depolarizer which is there to react with the hydrogen which would otherwise be generated by the chemical reaction with the electrolyte as the cell discharges.
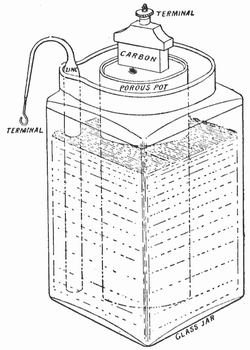
The chemical reaction turns the manganese dioxide into manganese oxide or manganese hydroxide. The ammonium chloride turns into ammonia and water. Fortunately modern zinc based batteries have changed the chemicals so ammonium based chemicals are no longer used. For a rechargeable battery you need a chemical reaction which can be reversed by reversing the direction of current flow.
A rechargeable battery is also called a secondary cell or secondary battery. The lead-acid battery in cars is a common example although the lithium-ion battery is another common example. The lead acid battery is probably older than the Leclanche cell, having been invented in 1859. When discharged, both the positive and negative plates are lead sulfate. When charged, the positive plate becomes lead dioxide and the negative plate turns into pure lead. Lead acid batteries gradually lose their ability to take charge due to the crystallization of the lead sulfate. Rather than forming an amorphous layer some of the lead sulfate starts to form a crystal which won’t re-dissolve on recharging.
Lithium rechargeable batteries use various compounds such as lithium manganese oxide. Unlike the lead acid cell, a lithium cell will have dissimilar materials for the two electrodes. The key characteristic of rechargeable batteries is that the chemical reaction can be reversed. There is also usually some unwanted deterioration of either the electrodes and/or the electrolyte with time and charge/discharge which is why they never last forever and usually have a noticeable loss of capacity after 500 or 1000 charge/discharge cycles.
A super capacitor is capacitor which has a massive capacitance – several Farads – and can therefore store a large amount of charge, much like a battery. The disadvantage is that they are currently expensive and the voltage will drop as the capacitor is discharged, unlike an “ideal” battery which maintains a relatively constant voltage until it is almost completely discharged. This voltage drop with the capacitor means that some electronics is almost always required to convert the voltage into something more stable for electronic products. However, they can last for 100,000 cycles or more compared to only 1000 cycles for rechargeable batteries.
So, coming back to the saltwater battery, are you really running it on salt water? Well, yes and no. The salt water is the electrolyte but there will also be some electrodes in there. While details are limited, the web site says that the anode will last 6 months. Remember, this is a primary cell so a chemical reaction is happening which cannot be reversed by reversing the direction of flow of the current. Unfortunately it is not just the salt water that deteriorates. However, you only throw away some of the battery when it is discharged so in that sense it creates less waste than a normal primary battery.


Leave a Reply