The heart of a flow battery is a specially designed regenerative fuel cell module. A conventional regenerative fuel cell operates on the basis of a reversible electrolysis electrochemical process and can be either an open system (where water can be added, and hydrogen and oxygen removed) or a closed system. These electrolysis-based regenerative fuel cells are generally limited to a few kilowatts of power. Flow batteries operate on different electrochemical processes and are more scalable than conventional regenerative fuel cells. Flow batteries can be several megawatts in size.
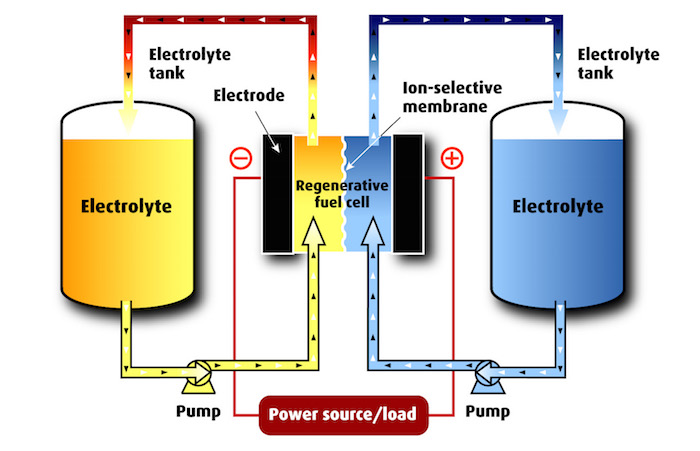
Flow batteries operate on the basis of various redox (reduction / oxidation) chemical reactions in which electrons are transferred between chemicals in different oxidation states. While there are several possible chemicals that can be used to fabricate flow batteries, today’s commercial flow batteries generally use iron-chloride, zinc-bromine or vanadium.
Both the iron-chloride and zinc–bromine flow batteries can be regarded as electroplating machines. During charging, iron or zinc is electroplated onto conductive electrodes. During discharge, the reverse process occurs: the metallic iron or zinc plated on the negative electrode dissolves in the electrolyte and is available to be plated again at the next charge cycle. Both these chemistries can be left fully discharged for extended periods without damage. As discussed below, Vanadium flow batteries operate on a different chemical basis.
Iron-chloride

Iron flow battery (IFB) technology uses iron in an electrolyte for reactions including a negative electrode where plating occurs, also referred to as the plating electrode, and a positive electrode where a redox reaction occurs, sometimes referred to as the redox electrode. The performance of an IFB battery can be broken down to its plating electrode performance (negative electrode), redox electrode performance (positive electrode), and ohmic resistance loss. On the plating electrode, the ferrous (Fe2+) ion gains electrons and plates as solid iron on the substrates during charge, as shown in the figure above, and the solid iron dissolves as ferrous ions and releases two electrons during discharge. The equilibrium potential for the iron plating reaction is -0.44V.
On the redox electrode, the redox reaction between ferrous and ferric (Fe3+) ions occurs during charge and discharge. On the positive electrode, two Fe2+ ions lose two electrons to form Fe3+ ions during charge and two Fe3+ ions gain two electrons to form Fe2+ during discharge. The equilibrium potential between ferrous and ferric ions is +0.77V. The reaction in an IFB redox flow battery is reversible.
Zinc-bromine
During charge of a zinc-bromine flow battery, metallic zinc is plated as a thick film on the anode side of a carbon-plastic composite electrode, and bromide ions are oxidized to bromine and evolved on the other side of the membrane. During discharge, the zinc metal is oxidized to Zn2+ ion and dissolved into the aqueous anolyte. Two electrons are released at the anode the external circuit. The electrons return to the cathode and reduce bromine molecules (Br2) to bromide ions, which are soluble in the aqueous catholyte solution. The bromine in the catholyte is converted into two bromide (Br-) ions at the cathode, balancing the Zn2+ cation and forming a zinc bromide solution. The chemical process used to generate the electric current increases the zinc-ion and bromide-ion concentration in both electrolyte tanks.

Vanadium
Vanadium is an unusual element. It can exist as several ions of different charges in solution, V(2+,3+,4+,5+), each having different numbers of electrons around the nucleus. Fewer electrons results in a higher positive charge. In a vanadium flow battery energy is stored by providing electrons making V(2+,3+), and energy is released by losing electrons to form V(4+,5+). As a result, different oxidation states of dissolved vanadium ions in the electrolyte (V2+, V3+, V4+, V5+) store or deliver electric energy.
The electrolyte is continuously fed from a tank system into the reaction cell. Depending on the current demand, energy is stored in the electrolyte (battery charging) or fed into the grid/network (battery discharging). Because the electrolyte flow direction does not have to change when loading and unloading, the VRFB vanadium flow battery from Schmid is very fast in the response time (less than 20ms). It can be switched between charging and discharging under full load. When the system is in standby mode, only a minimum discharge of the stack takes place. In the electrolyte tank there is no discharge taking place until the electrolyte again flows through the stack by turning on the pumps.
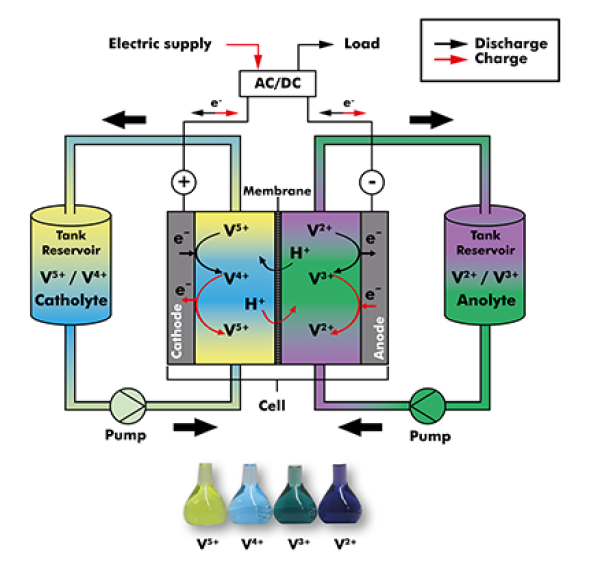
In a Vanadium redox flow battery, only one element (Vanadium) in the electrolyte is used for storing energy. (Source: Schmid)
Part two of this three-part FAQ series will discuss, “Flow Batteries – What Can You Use Them For?”
References:
Energy Storage, Electrosynthesis
ESS Energy Warehouse, ESS, Inc.
Flow Battery, Wikipedia
Vanadium Redox Flow Battery, Schmid
Zinc-Bromine Flow Battery, Redflow


Leave a Reply